Alkene Alkyne Naming Priority Rules
- Alkene or Alkyne whichever has the lower locant (carbon number) gets alkene/alkyne naming priority
- When the alkene and alkyne locant numbers are the same (tie), the alkene gets the priority of numbering.
- The substituent naming is based on alphabetical for alkene and alkyne. i.e. –ene- comes before –yne.
- If there is a functional group or groups with higher priority, the higher–priorty functional group takes the name of the main chain, which comes to the last part of name.
This is not limited Alkene or Alkyne. The general rule of IUPAC.
IUPAC organic chemistry generally does not allow repeating vowels between functional group names. The preceding vowel is omitted when it is followed by another vowel.
e.g.
Correct: but-2-en-1-ol
Wrong: but-2-ene-1-ol
But it allows repeating bowels between number prefix (di, tri, tetra, hepta, etc…) and names.
e.g.
Correct: 2,2,3,3-tetraaminobutan-1-ol
Wrong: 2,2,3,3-tetraminobutan-1-ol
Correct: (E)-2,3-diaminobut-2-en-1-ol
Wrong: (E)-2,3-daminobut-2-en-1-ol
Alkene Alkyne Naming Priority Example 1
Case 1: Alkene and Alkyne have the different locant (carbon number)
In this case, alkene and alkyne have different locant (carbon number) depending on where to start counting.
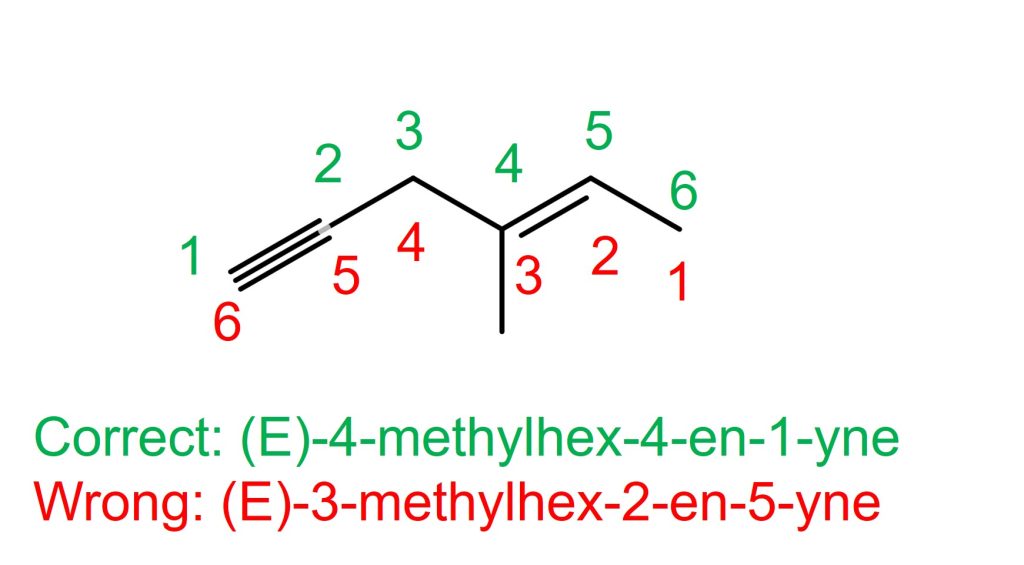
The left molecule in the above example
The alkyne, is on C1-C2 if counted from the left. The alkene is on C2-C3 if counted from the right. Therefore, the alkyne with C1 takes the priority. The correct name is (E)-4-methylhex-4-en-1-yne. NOT (E)-3-methylhex-2-en-5-yne.
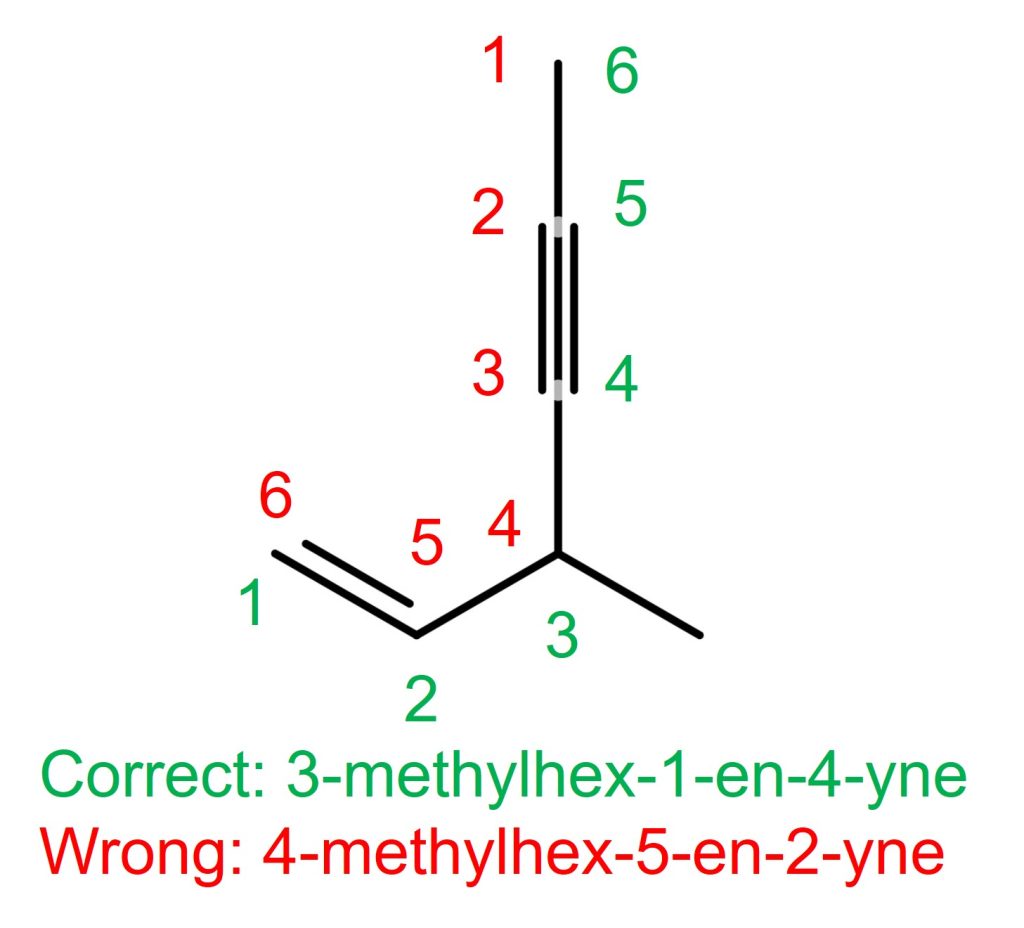
The right molecule in the above example
The alkyne, is on C2-C3 if counted from the top. The alkene is on C1-C2 if counted from the left. Therefore, the alkene with C1 takes the priority. The correct name is 3-methylhex-1-en-4-yne. NOT 4-methylhex-5-en-2-yne.
Alkene Alkyne Naming Priority Example 2
Case 2: Alkene and alkyne have the same locant (carbon number)
In this case, alkene and alkyne have the same locant (carbon number) depending on where to start counting.
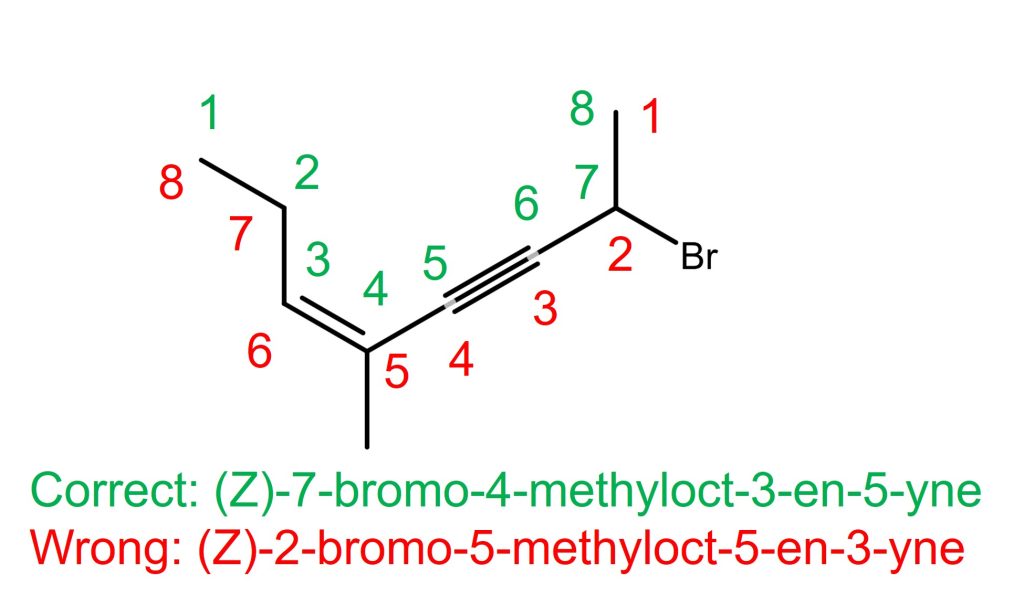
The left molecule in the above example.
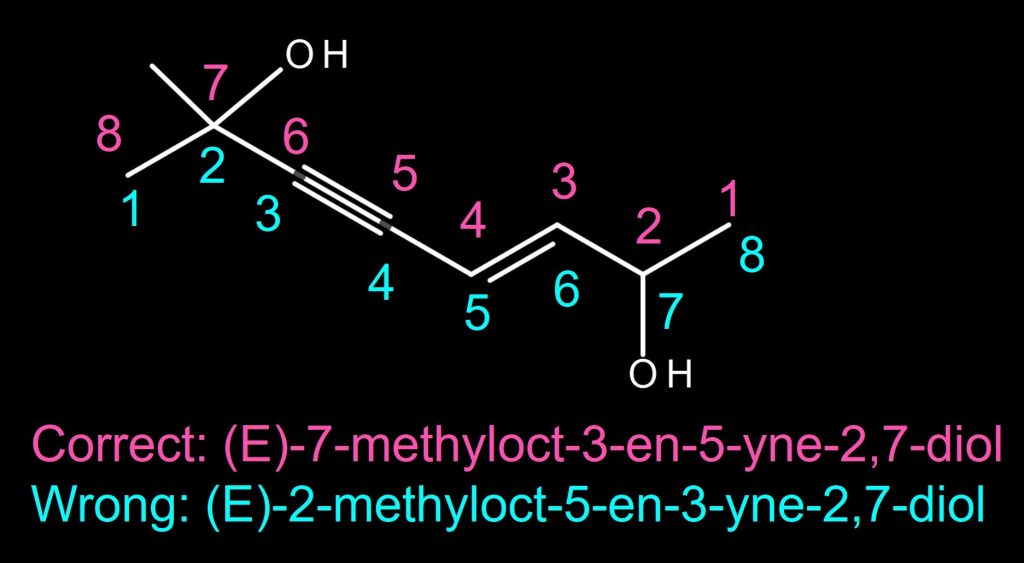
The alkene is on C3-C4 if counted from the left, and the alkyne is also on C3-C4 if counted from the right. In this case, the alkene takes priority over the alkyne according to the tiebreaker rule specified in the IUPAC Blue Book 2013 edition, P-31.1.1.1. Therefore, the alkene with C3 takes the priority. The correct name is (Z)-7-bromo-4-methyloct-3-en-5-yne. NOT (Z)-2-bromo-5-methyloct-5-en-3-yne. The stereochemistry of the alkene is “Z” and it doesn’t need the locant on “Z” or 3Z because there is only one alkene in the molecule. The order of bromo and methyl group substituents is based on the alphabetical order in the molecule name.
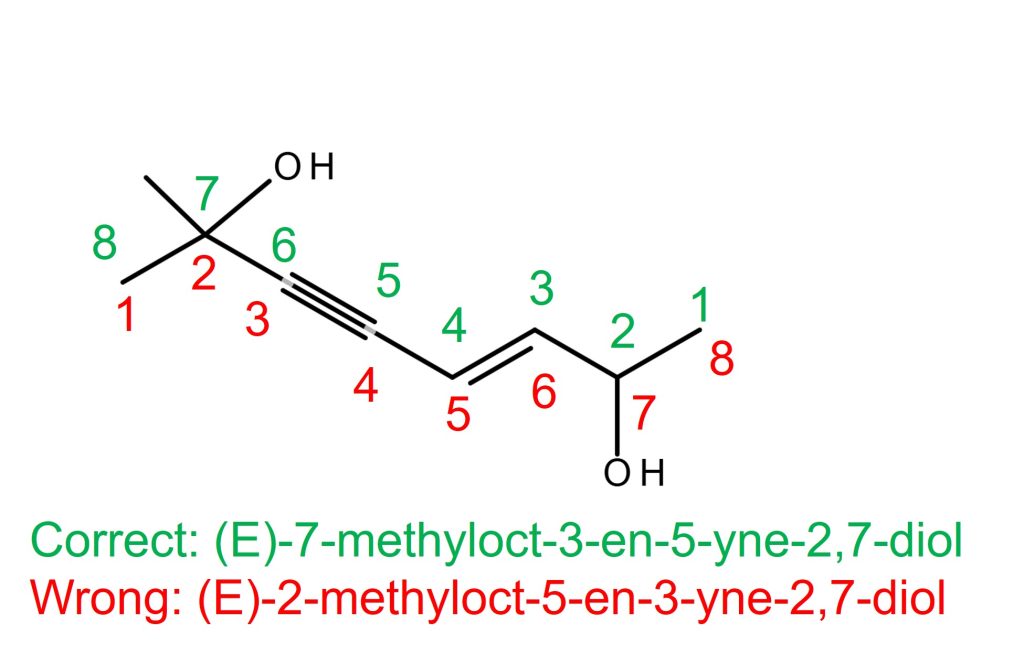
The right molecule in the above example.
The alkene is on C3-C4 if counted from the right, and the alkyne is also on C3-C4 if counted from the left. In this case, alkene takes priority over alkyne according to the tiebreaker rule. Therefore, the alkene with C3 takes the priority. The correct name is (E)-7-methyloct-3-en-5-yne-2,7-diol. NOT (E)-2-methyloct-5-en-3-yne-2,7-diol. The stereochemistry of the alkene is “E”, and it doesn’t need the locant on “E” because there is only one alkene in the molecule. The alcohol has a higher priority than alkene or alkyne. Therefore, “-en-” and “-yne-” come before “-diol” in the main chain name even though “-diol” is alphabetically earlier than “-en-“.
Alkene Alkyne Naming Priority Example 3
Case 3: The main chain doesn't have the longest chain
What if the longest chain doesn’t contain the alkene or alkyne?
The organic chemistry nomenclature rules come from the IUPAC Blue Book, which was originally published in 1979. It was updated in 1993 (old) and 2013 (current). In the 1993 guideline, the greater number of multiple bonds had higher priority than the greater number of skeletal atoms for naming the main chain. However, this priority is reversed in the 2013 (latest) edition.
The IUPAC Blue Book 2013 edition P-46.1 (b) states
“In acyclic substituents the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred acyclic substituent is the length of the chain; unsaturation is now a lower criterion [see (d)].“
Therefore, the longest chain rule gets higher priority than the chain with the greater number of multiple bonds.
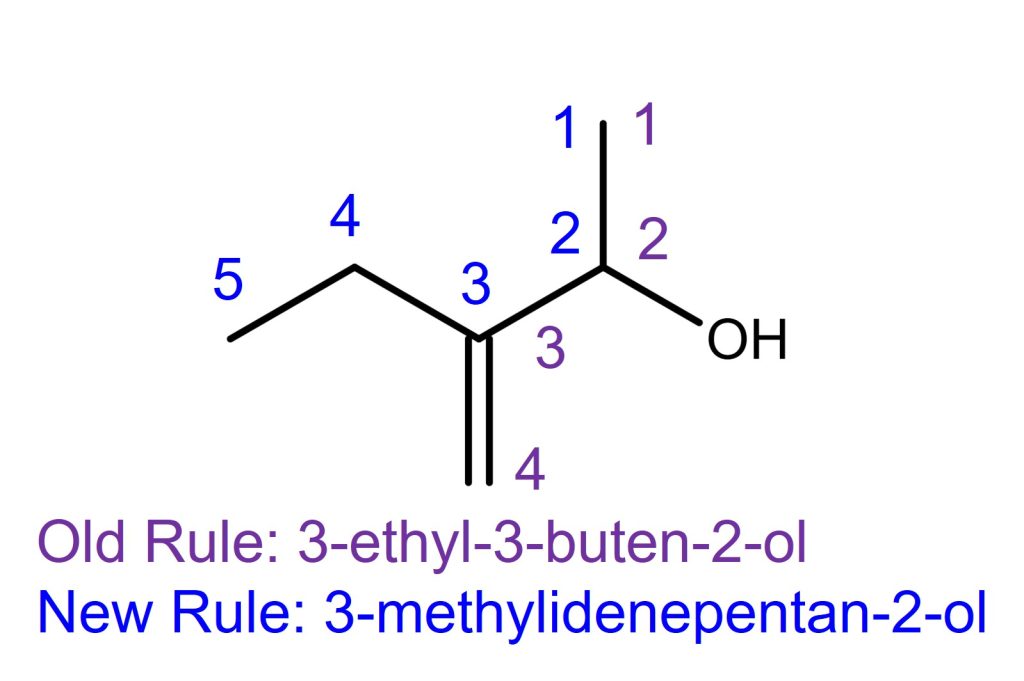
The Left molecule in the above example.
The alkene substituent is on C3. The locant count starts from the top because the alcohol has a higher priority than the alkene.
Per the IUPAC Blue Book 1993 (old) edition rule,
the main chain ends at the alkene. The old rule name is 3-ethyl-3-buten-2-ol.
Per the IUPAC Blue Book 2013 (current) edition rule,
the longest carbon chain rule supersedes the chain with the greatest number of multiple bonds. Therefore, the locant number starts at the top and ends at the left. The main chain has 5 carbons. The IUPAC name for this is 3-methylidenepentan-2-ol. Methylidene is the single carbon substituent with a double bond. The alkene doesn’t need E or Z notation because it’s connected to 2 hydrogens; therefore there is no stereochemistry.
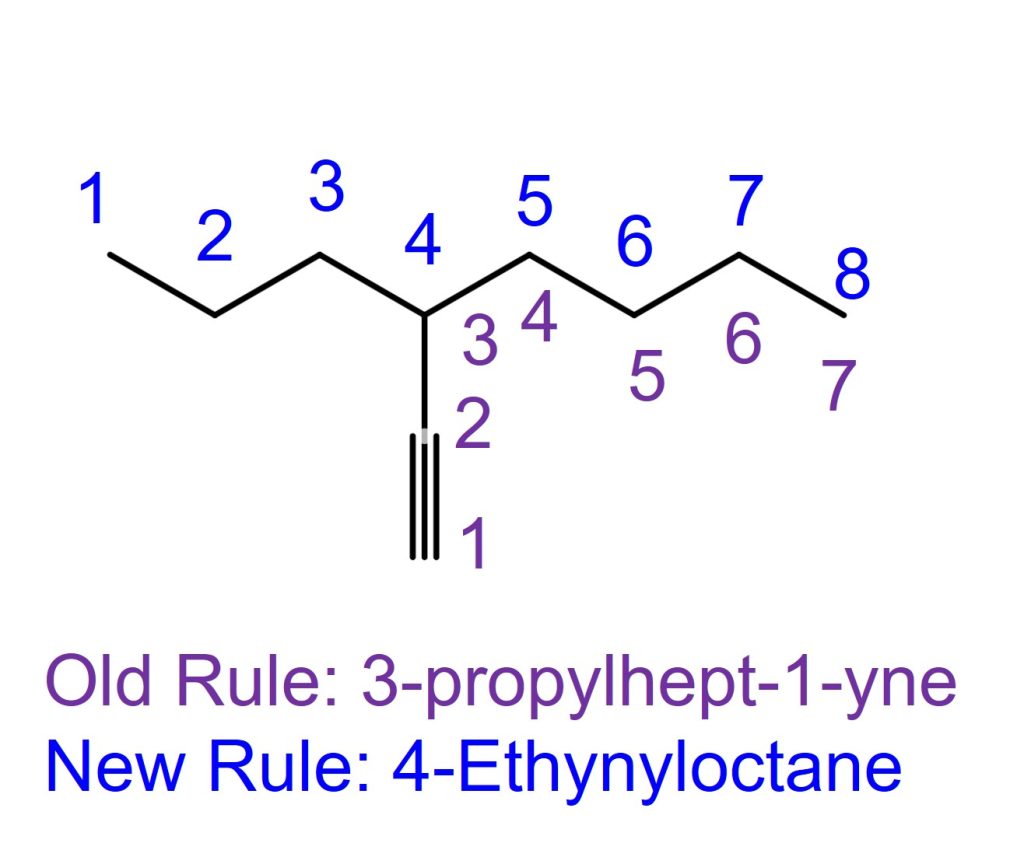
The right molecule in the above example.
Per the IUPAC Blue Book 1993 (old) edition rule,
the counting of the locant starts at the alkyne. Then count the carbon, and it’ll be a 7-carbon main chain. The old rule name is 3-propylhept-1-yne.
Per the IUPAC Blue Book 2013 (current) edition rule,
the longest carbon chain rule supersedes the chain with the greatest number of multiple bonds. Therefore, the locant number starts at the left, which gives the ethynyl substituent a lower locant number than the one counting from the right. The IUPAC name for this is 4-Ethynyloctane. Ethynyl is the 2-carbon substituent with a triple bond on the first and second carbon of the substituent.
For the exam,
Ask the instructor for the clarification that either or both forms will be accepted.