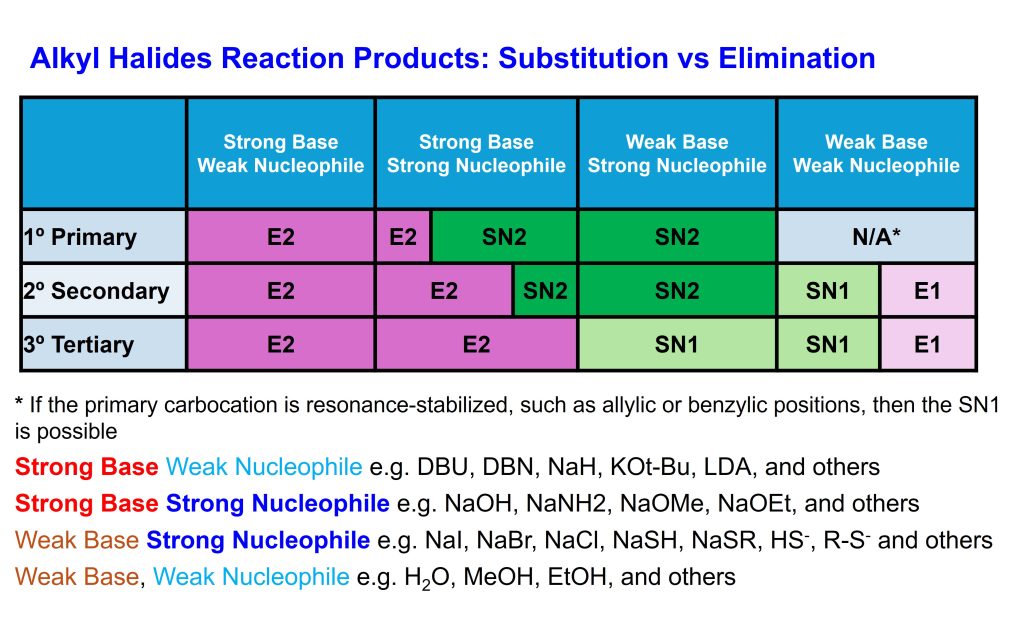
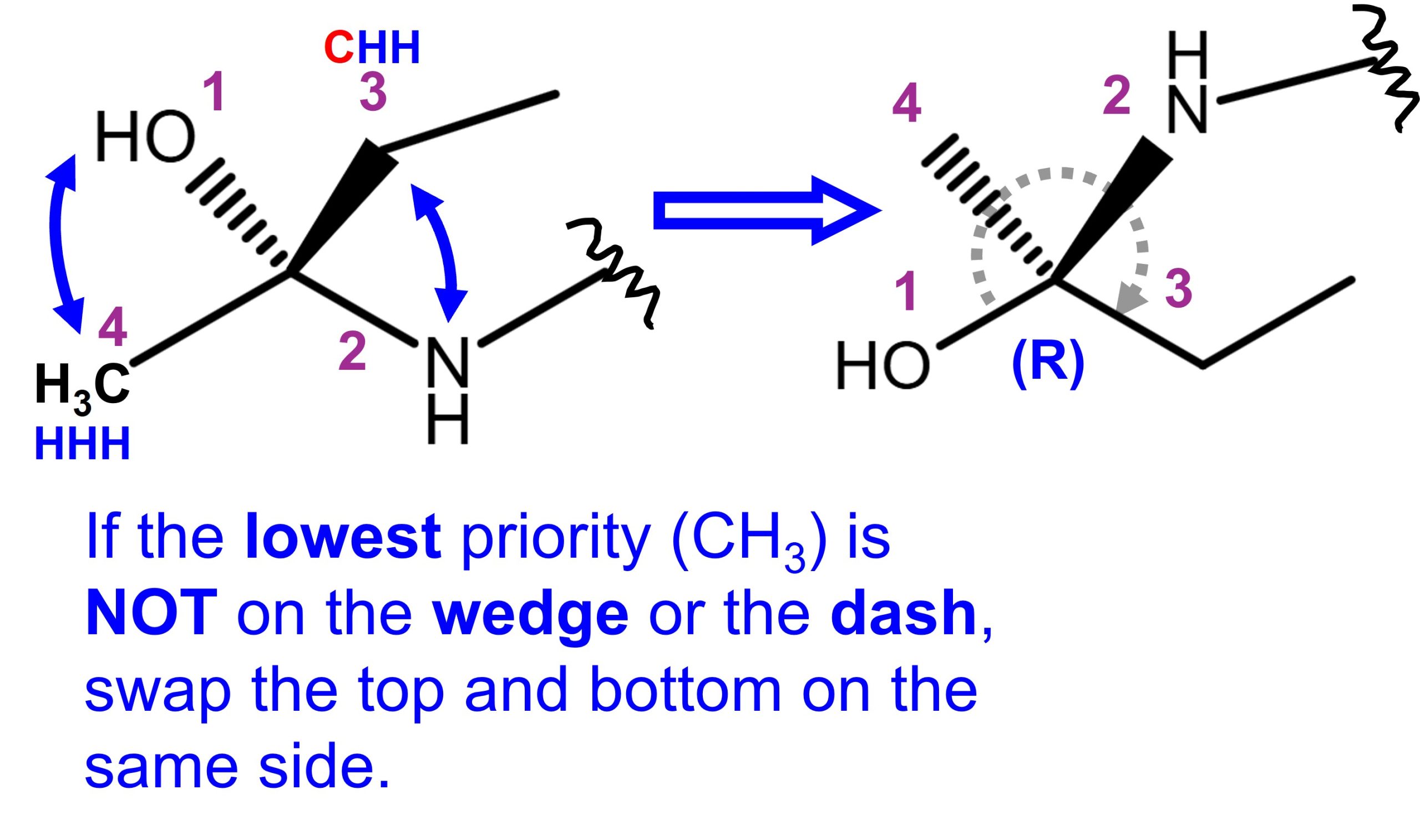
Characteristics of SN2 SN1 E2 E1 Reactions based on strong/weak bases or nucleophiles and the likelihood of product formation based on the substitution of carbons.
SN2: Substitution Nucleophilic Bimolecular
SN1: Substitution Nucleophilic Unimolecular
E2: Elimination Bimolecular
E1: Elimination Unimolecular
SN2/SN1 Substitution Reaction Summary
SN2 Reaction
- Strong nucleophile
- Polar aprotic solvent
- Prefers the least substituted carbon
- No carbocation formation
- Concerted (happens in 1 step)
- 1 activation energy peak since concerted step
- Rate = k*[alkyl halide]*[nucleophile]
- Second order reaction
- Cold Temperature favors SN2 over E2 or SN1 with strong nucleophile except for tertiary carbon.
- Bulky base (srong/weak) is normally NOT used for SN2
- Inversion of Stereochemistry (Backside attack): stereochemistry of added nucleophile is the opposite of leaving group.
SN1 Reaction
- Weak Base
- Polar protic solvent
- Prefers the most substituted carbon
- Carbocation formation at most substituted carbon
- Multi-step reaction
- 2 activation energy peaks
- Rate = k*[alkyl halide]
- First order reaction
- Cold Temperature favors SN1 over E1 with weak nucleophile and weak abase.
- Bulky base (srong/weak) is normally NOT used for SN1
E2/E1 Elimination Reaction Summary
E2 Reaction
- Strong Base
- Polar aprotic solvent
- Strong small base prefers the most substituted double bond (Zaitsev product)
- Strong bulky base prefers the least substituted double bond (Hofmann product)
- No carbocation formation
- Concerted (happens in 1 step)
- 1 activation energy peak since concerted step
- Rate = k*[alkyl halide]*[base]
- Second order reaction
- Hot Temperature favors E2 over SN2 with strong base
- Leaving halogen and hydrogen must be in antiperiplanar position
E1 Reaction
- Weak Base AND Weak Nucleophile
- Polar protic solvent
- Prefers the most substituted
- double bond (Zaitsev product)
- Carbocation formation at most substituted carbon
- Multi steps
- 2 activation energy peaks
- Rate = k*[alkyl halide]
- First order reaction
- Hot Temperature favors E1 over SN1 with
- weak base AND weak nucleophile
SN2 Reaction
Srong Base Strong Nucleophile
e.g. NaOH, NaNH2, alkoxides (NaOMe, NaOEt) etc…
Primary carbon:
The majority of reaction is SN2. The minority is E2
Secondary carbon:
The majority of reaction is E2. The minority is SN2
Tertiary carbon:
Entire reaction is E2
Weak Base Strong Nucleophile
e.g. NaI, NaBr, NaCl, NaSH, NaSR, HS–, R-S–, etc…
Primary carbon:
The almost entire reaction is SN2
Secondary carbon:
The almost entire reaction is SN2
Tertiary carbon:
Entire reaction is SN1
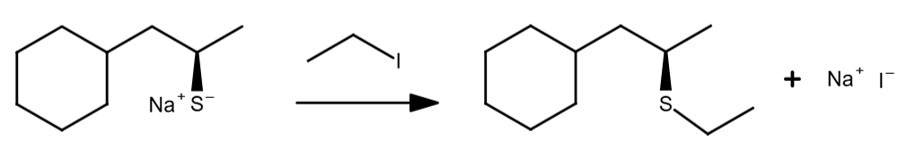
SN2 Reaction Examples
SN1 Reaction
Weak Base Strong Nucleophile
e.g. NaI, NaBr, NaCl, NaSH, NaSR, HS–, R-S– etc…
Primary carbon:
No SN1 reaction (instead, SN2)
Secondary carbon:
No SN1 reaction (instead, SN2)
Tertiary carbon:
SN1
Weak Base Weak Nucleophile
e.g. water and alcohols (H2O, MeOH, EtOH), etc…
Primary carbon:
Generally no SN1 for primary carbocation*
Secondary carbon:
The Low temperature preferes SN1.
The high temperature and/or poor nucleophile prefers E1.
Tertiary carbon:
The Low temperature preferes SN1.
The high temperature and/or poor nucleophile prefers E1.
* If the primary carbocation is resonance-stabilized, such as allylic or benzylic positions, then the SN1 / E1 is possible. The cold temperature prefers the SN1 and the hot temperature prefers E1.
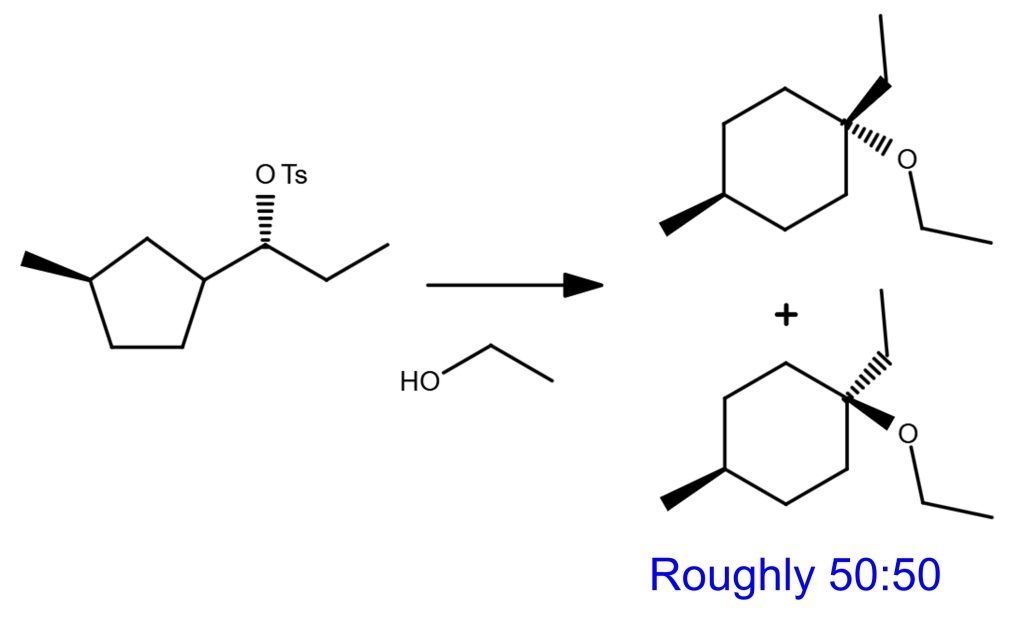
SN1 Reaction Example
E2 Reaction
Srong Base Weak Nucleophile
e.g. Bukly bases (DBU, DBN, KOt-Bu, LDA), non-nucleophile (NaH), etc…
Primary carbon:
All E2
Secondary carbon:
All E2
Tertiary carbon:
All E2
Srong Base Strong Nucleophile
e.g. NaOH, NaNH2, alkoxides (NaOMe, NaOEt) etc…
Primary carbon:
The majority of reaction is SN2. The minority is E2
Secondary carbon:
The majority of reaction is E2. The minority is SN2
Tertiary carbon:
Entire reaction is E2
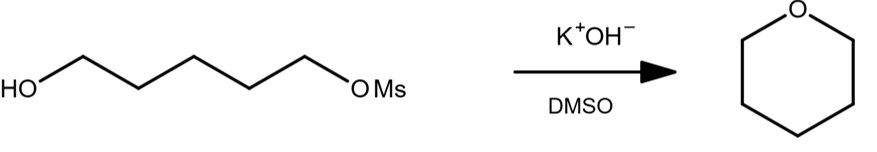
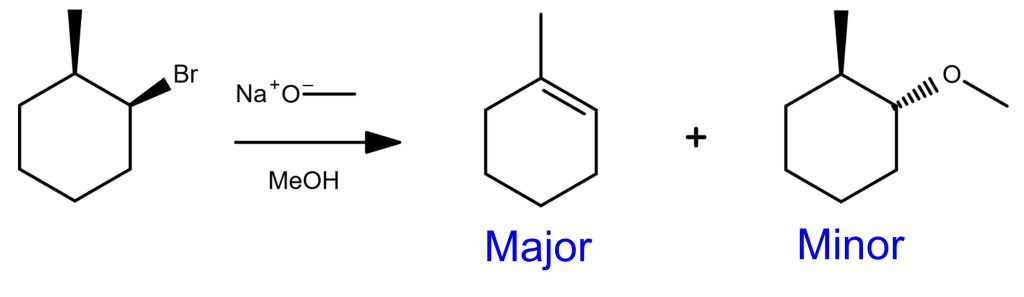
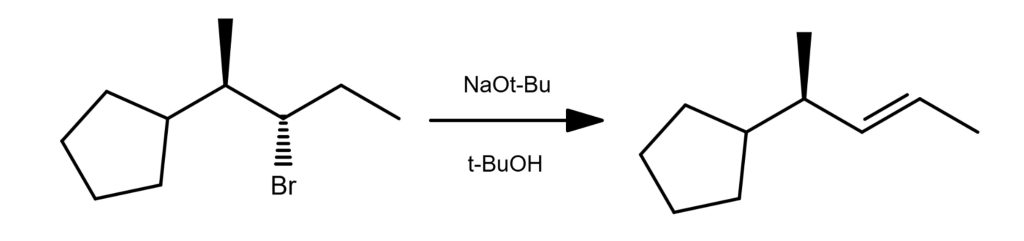
E2 Reaction Examples
E1 Reaction
Weak Base Weak Nucleophile
e.g. water and alcohols (H2O, MeOH, EtOH), etc…
Primary carbon:
Generally no SN1 / E1 for primary carbocation*
Secondary carbon:
The Low temperature preferes SN1.
The high temperature and/or poor nucleophile prefers E1.
Tertiary carbon:
The Low temperature preferes SN1.
The high temperature and/or poor nucleophile prefers E1.
No Other Type of Reagents
* If the primary carbocation is resonance-stabilized, such as allylic or benzylic positions, then the SN1 / E1 is possible. The cold temperature prefers the SN1 and the hot temperature prefers E1.
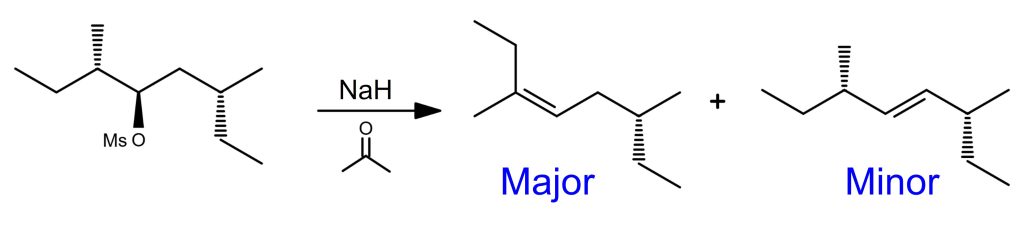
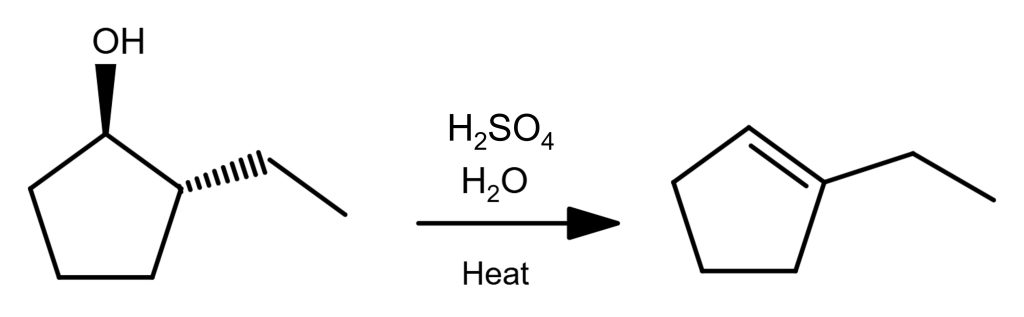
E1 Reaction Examples
DBU: 1,8-diazabicycloundec-7-ene (DBU)
DBN: 1,5-diazabicyclonon-5-ene (DBN)
NaH: Sodium Hydride
KOt-Bu: Potassium tert-butoxide
LDA: Lithium Diisopropylamide