R S Configuration of Fischer Projection
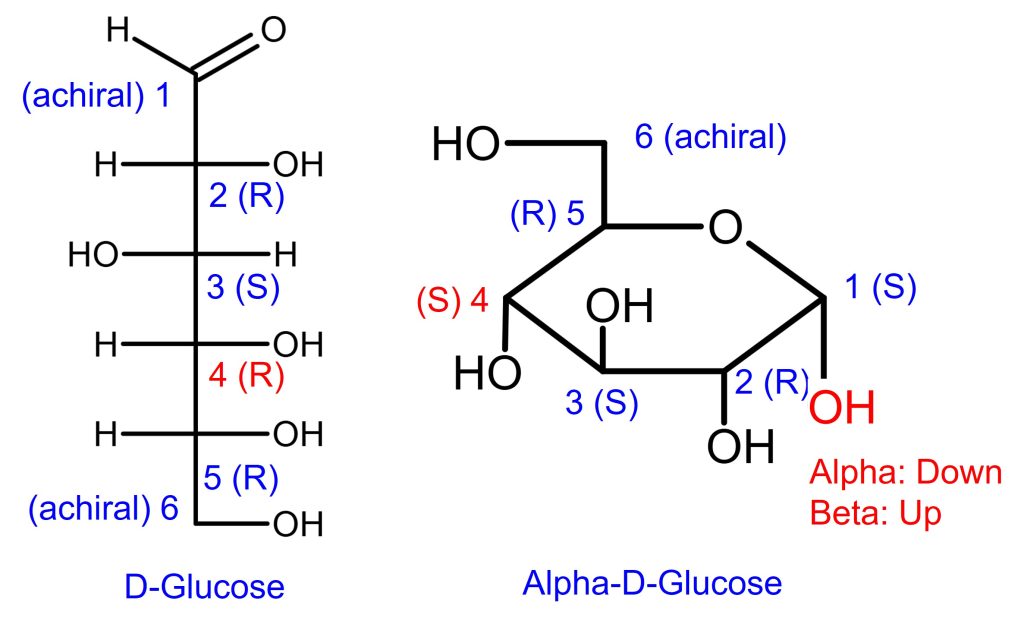
Example D-Glucose and L-Glucose
We’re going to work on Fischer Projection before R S Configuration for Haworth Projection.
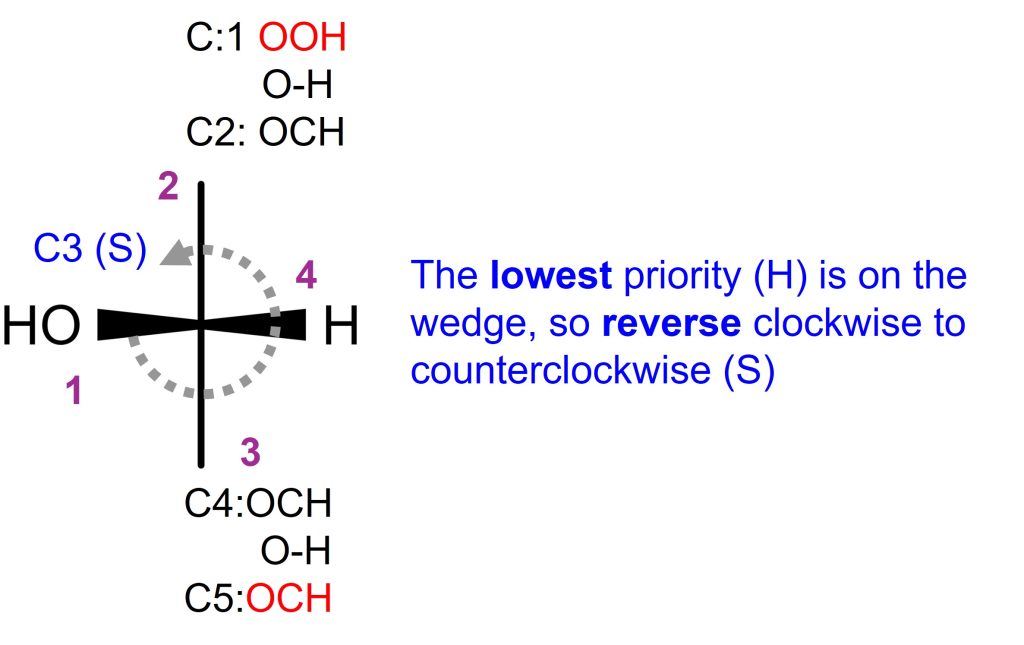
Let’s take a look at C3 of D-glucose.
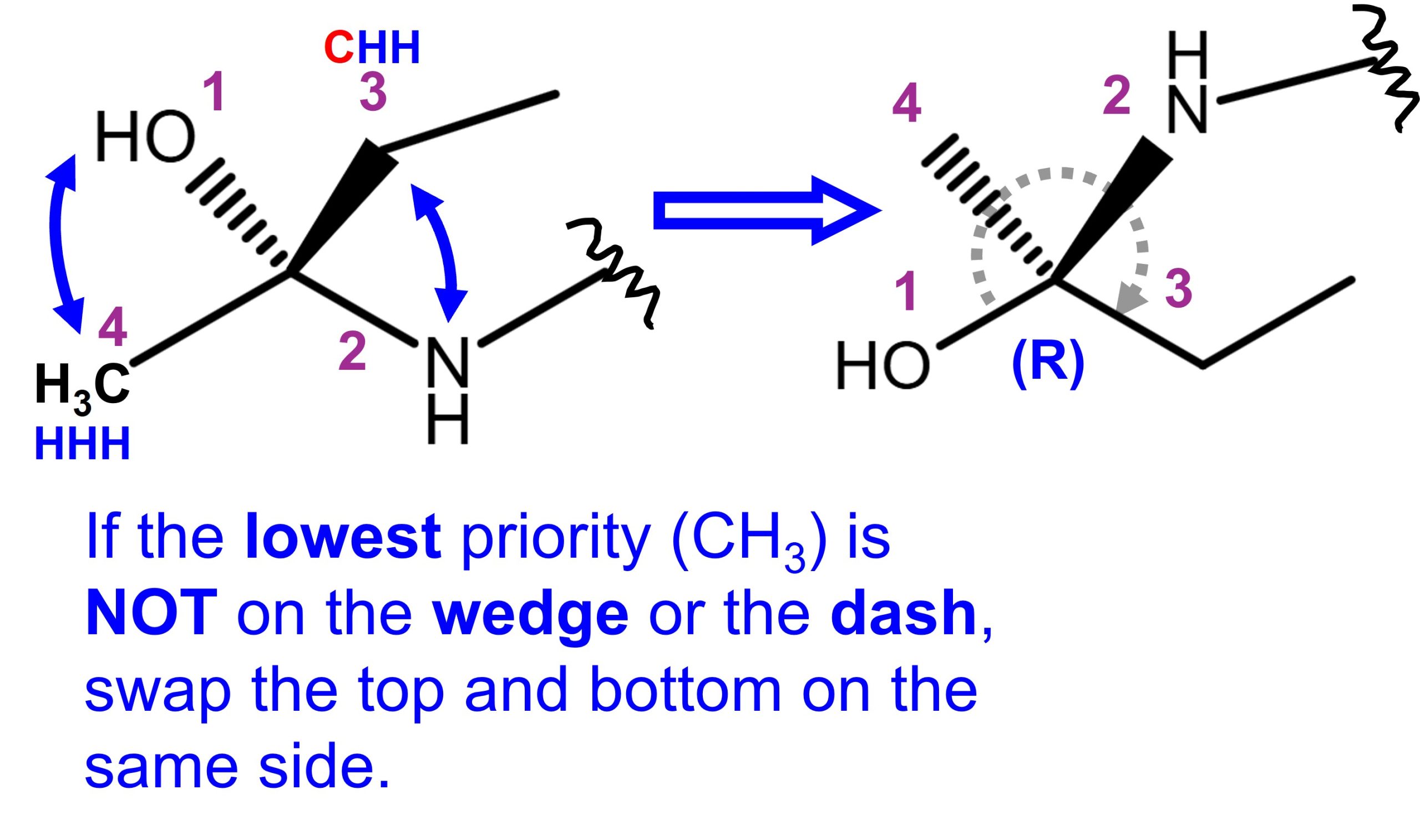
In the Fischer projection, the horizontal line is the wedge. Identify the priority according to Cahn-Ingold-Prelog rules.
- First, Identify C3 as chiral
- Next, C3 is connected to O, C, C, H. Priority 1 is O, and Priority 4 is H. Two carbons (C2, C4) are tie.
- Then, look at what is connected to C2 and C4.
- C2 is connected to O, C (C1), H. C4 is also connected to O, C (C5), H. They’re still tie.
- Oxygen is the highest priority among O, C, H. So, look at what is connected to the C2 and C4 oxygen. They are both connected to H, and that’s where the line ends.
- So, move on to the next highest priority from C2 and C4. Among O, C, and H, C2 is connected to C1, and C4 is connected to C5.
- C1 is connected to O, O (carbonyl double bond), H. C5 is connected to O, C, H. Now the tie is broken. C1 wins by one extra oxygen.
- Therefore, C2 becomes the second priority, and C4 becomes the third priority.
- The circle draws the clockwise (CC) around C3. But since the lowest priority (hydrogen) of C3 is on the wedge, reverse the rotation, thus, it becomes counterclockwise (CCW). Therefore, C3 is (S).
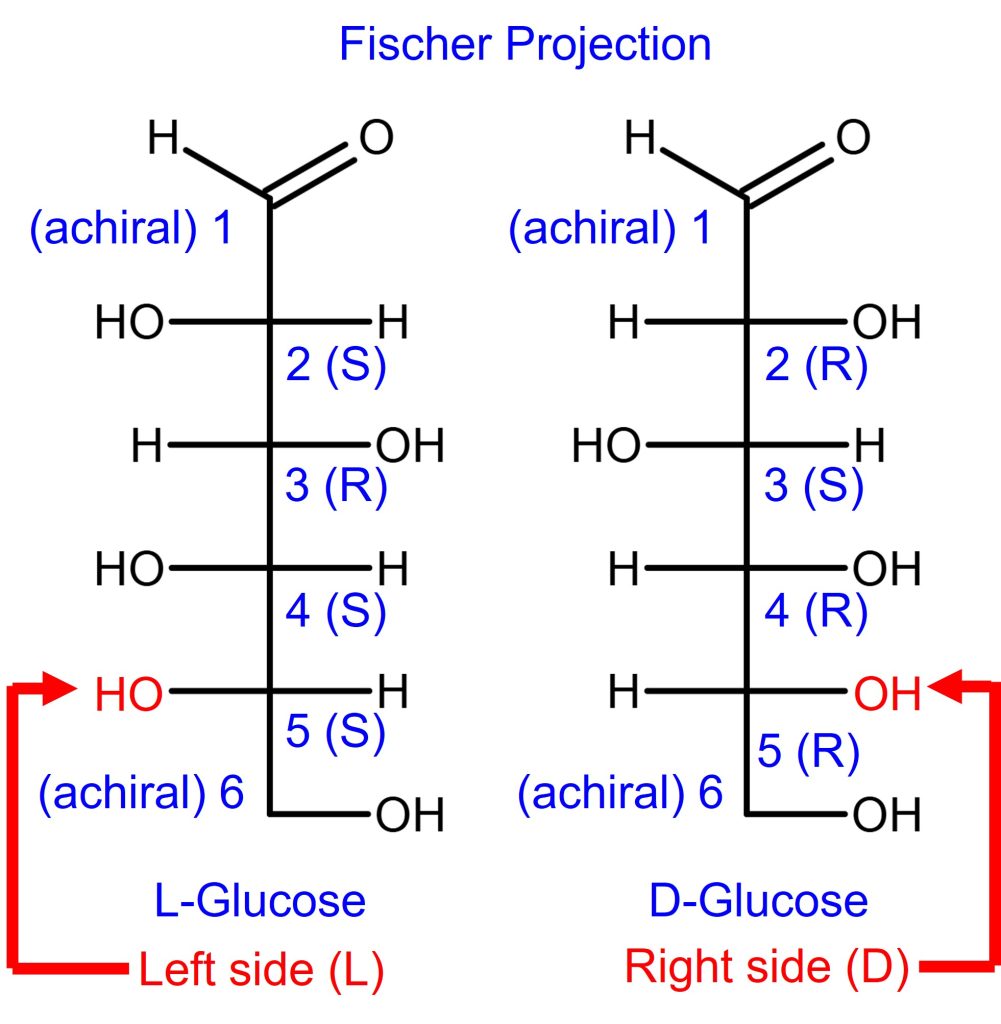
In addition, the carbons in L-glucose are the opposite of D-glucose. The answer key is shown above for your own practice.
How to identify D-Glucose or L-Glucose optical rotation
The way to identify the D/L optical rotation of a Fischer projection of a monosaccharide is to look at the stereochemistry (R/S) of the farthest chiral carbon from the carbonyl carbon.
- If (R) or -OH is on the right side, that’s D-glucose.
- If (S) or -OH is on the left side, it is L-glucose.
- Keep in mind, the R/S designation does NOT automatically determine D or L.
- For monosaccharide Fischer projection, it happens to be that way.
R S Configuration of Haworth Projection
Example D-Glucose

R S Configuration Haworth Projection
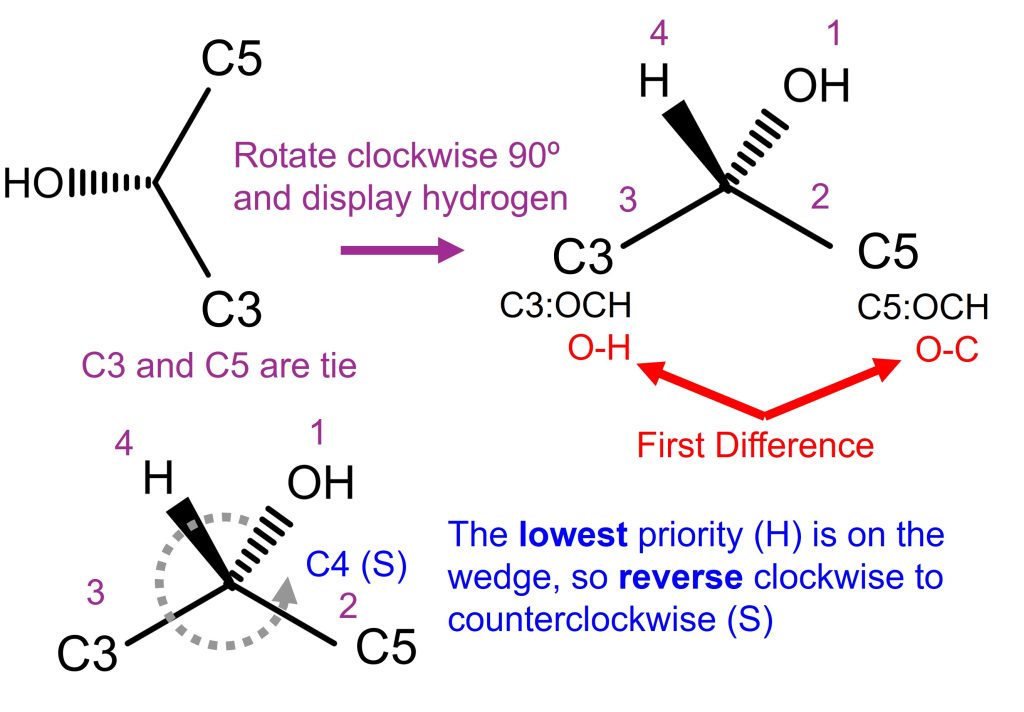
When the Fischer projection of glucose becomes the Haworth projection, it becomes glucopyranose and the C4 stereochemistry changes. Let’s look at the C4 in the above figure. In the above figure, C4 is isolated and rotated for ease of understanding.
- First, C4 is connected to O, C3, C5, H. O is the first priority, and H is the 4th (lowest) priority. C3 and C5 are the tie.
- Look at the connection of C3 and C5. C3: OCH, C5: OCH. They’re still tie.
- Now, look at the connection of the highest priority connected to C3 and C5. They’re oxygens.
- C3 oxygen is connected to H (OH). C5 oxygen is connected to C1. Therefore, C5 takes priority.
- The priority of atoms connected to C4 is O (1st), C5 (2nd), C3 (3rd), and H (4th). It makes a clockwise rotation.
- Since the lowest priority (hydrogen) is on the wedge, reverse the clockwise to counterclockwise, which makes it (S).
How to identify Alpha or Beta Designation in Haworth Projection
For C1 (anomeric carbon), if OH is in the down position, it’s alpha and (S). If OH is in the up position, it’s beta and (R). Keep in your mind, it’s determined based on the relationship with atoms that are connected to C1 carbon. Alpha and beta designation does NOT automatically determine (R) or (S) stereochemistry. Carbons in L-glucose have exactly opposite (R,S) stereochemistry.
The answer key for R S Configuration Haworth Projection is shown above for your own practice.