Chiral Center
- The chiral center is a type of stereocenter.
- It can be any atom (carbon is most common).
- It is sp3 hybridized* (e.g. tetrahedral, trigonal pyramidal etc..).
- It can have lone pairs.
- It cannot be planar geometry.
- The substituents need to be all different atoms or groups. i.e. it cannot be achiral.
- It cannot have a pi bond (double or triple bond).
*Under rare exceptions, the hybridization other than sp3 can be chiral.
Stereocenter
- It can be any atom (carbon is most common).
The hybridization can be either sp2 or sp3 but not sp. - It can have lone pairs.
- It can be planar geometry.
- The substituents need to be all different atoms or groups whether they are sp2 or sp3.
- It can have a double bond but not a triple bond.
- It can be chiral or achiral.
All chiral centers are stereocenters.
But not all the stereocenters are chiral centers.
Therefore, if the atom is not a stereocenter, it is not a chiral center.
Chiral Center vs Stereocenter Example 1
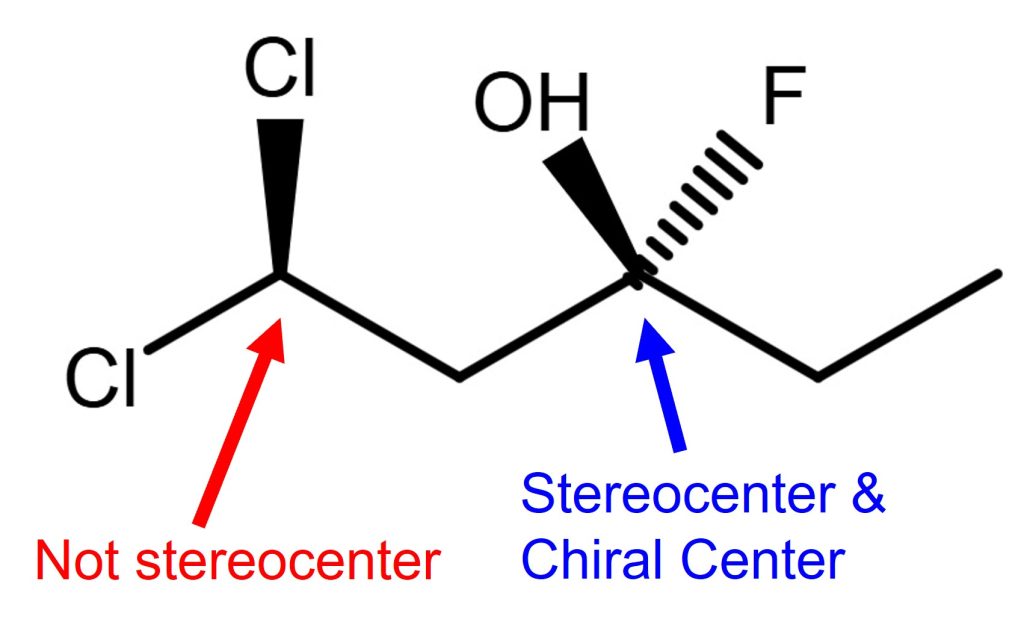
In example 1, the carbon on the left (C5, red labeled) is not a stereocenter because it is connected to 2 chlorine atoms, which are identical. This carbon can’t make a stereoisomer by swapping the position of substituents (connected atoms or the rest of the branch) to it.
The carbon on the right (C2, blue labeled) is a stereocenter and chiral center. Because this carbon is connected to all different substituents (OH, F, and 2 different hydrocarbon chains on the left and right sides). It can make different stereoisomers by swapping the positions of substituents. Also, it is a chiral center since it is sp3 and not planar geometry and has no pi bonds.
Chiral Center vs Stereocenter Example 2
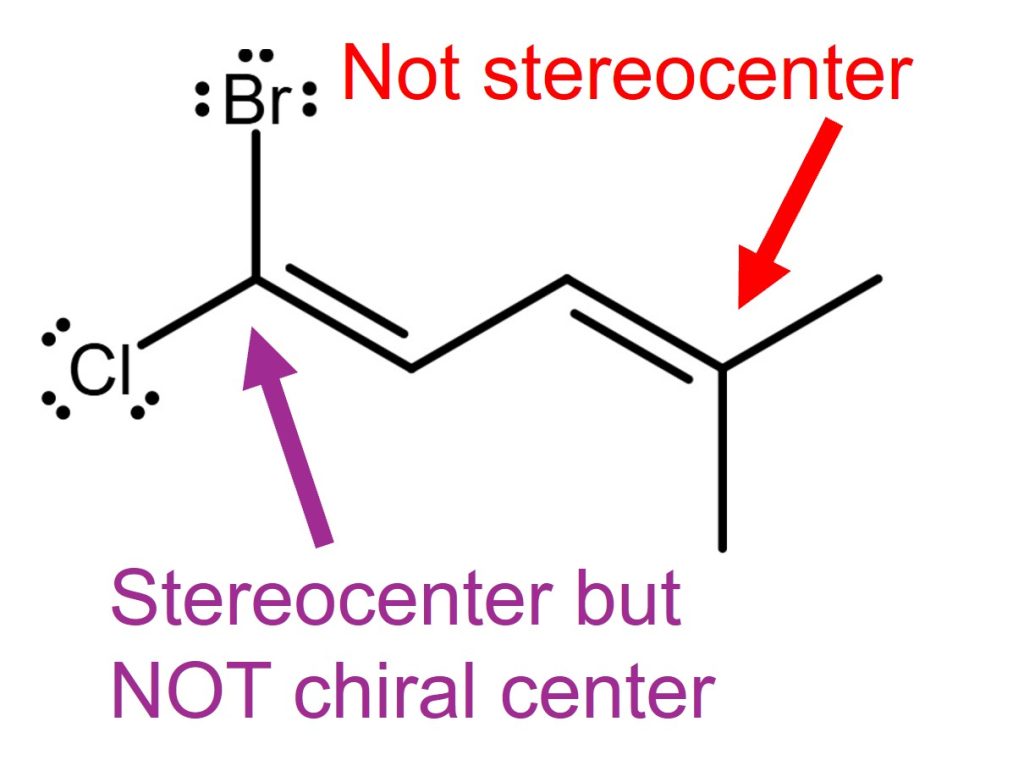
In example 2, the carbon on the right (C4, red labeled) is not a stereocenter. Because this carbon is connected to 2 methyl groups, it cannot make stereoisomers by swapping the substituents.
The carbon on the left side (C1, purple labeled), is a stereocenter but not a chiral center. Because it is connected to all different substituents. Since C2 carbon is connected to hydrogen and the rest of the carbon chain, an alkene by C1 and C2 can make stereoisomers (E or Z) by swapping the substituents connected to C1 or C2. See this for more about the stereocenter of alkene.
Since the C1 carbon has a trigonal planar geometry, it cannot be a chiral center.
Chiral Center vs Stereocenter Example 3
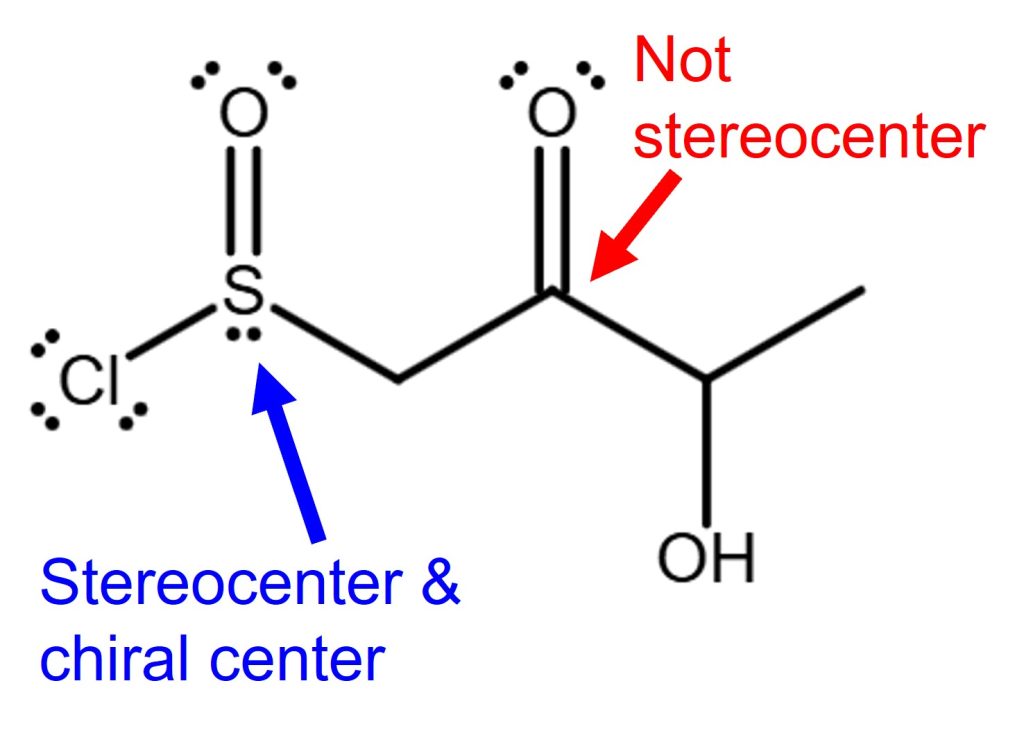
In example 3, the carbon on the right (C2, red labeled) is not a stereocenter. Because this carbon is in trigonal planar geometry, it cannot make stereoisomers by swapping the substituents.
For the sulfoxide sulfur on the left side (blue labeled), it is a stereocenter and also a chiral center. Because it is connected to all different substituents with a lone pair of electrons. It can make stereoisomers by swapping the substituents. It is a chiral center because it is sp3 hybridized and has a trigonal pyramidal geometry. It can form, for example, enantiomers by swapping the substituents.
Also check out
Stereocenters of alkene
Need online private tutor?
Contact us
What topics do you want to see? or any questions? Leave comments below.